XR-17 technology platform
Oasmia developed XR-17™ to address a problem that affects many promising medicines in development. They aren’t soluble in water. In fact, because of this flaw research on many of these drugs is halted long before they ever reach a patient.
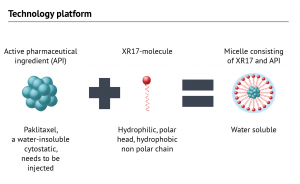
Oasmia’s XR-17 platform allows for formulations of active pharmaceutical ingredients that wouldn’t otherwise be water-soluble, so that these can be administered to patients.
An alternative to water is to use different carriers, for example polymers or oil derivatives. However, these carriers may cause adverse effects that can be severe. In cancer treatment, these side effects are often accepted in effective drugs because the other option is even worse—that patients aren’t treated at all.
Advantages of XR-17
XR-17™ technology can encapsulate individual active pharmaceutical ingredients as well as combinations of many ingredients with different solubility profiles. Oasmia’s toxicological and clinical studies have confirmed XR-17’s beneficial properties, leading to the following possible advantages:
- Improved solubility, which results in safer intravenous administration to patients
- Shorter infusion time, which makes the treatment more convenient for patients
- Reduced need for premedication because of the removed risk of serious hypersensitivity reactions against existing solvents such as CrEL and polysorbate 80